The Impact of IT Outage on Pharmacovigilance and the importance of having a robust IT system
August 19, 2024
On July 19th, a major IT outage caused by Microsoft disrupted various global sectors, including healthcare. This incident highlighted the critical role of reliable IT systems in pharmacovigilance. Pharmaconsulta, as a leading consultancy in pharmaceutical regulatory affairs in Malta, we are committed to maintaining uninterrupted operations despite this global disruption. In this article, we will explore the impact of IT outages on pharmacovigilance, detailing how robust IT infrastructure safeguards its operations and ensures drug safety.
Understanding Pharmacovigilance
Pharmacovigilance is essential for public health, involving systematically monitoring drug safety. Defined by the European Commission, it is the science and activities related to detecting, assessing, understanding, and preventing adverse drug reactions (ADRs) or other drug-related problems. The primary objectives of pharmacovigilance are to:
- Identify emerging safety concerns: Continuous monitoring helps spot potential safety issues early.
- Minimise risks: by assessing and mitigating risks associated with drug use.
- Ensure compliance: adherence to regulatory requirements and maintaining public trust in medicine safety.

IT systems are integral to pharmacovigilance, managing vast amounts of data related to adverse drug reactions (ADRs), patient information, and drug safety records. These systems streamline the collection, storage, and analysis of ADR reports, ensuring timely and accurate data processing. Electronic reporting systems, such as EudraVigilance in Europe, facilitate the efficient submission and tracking of safety data from healthcare providers and patients
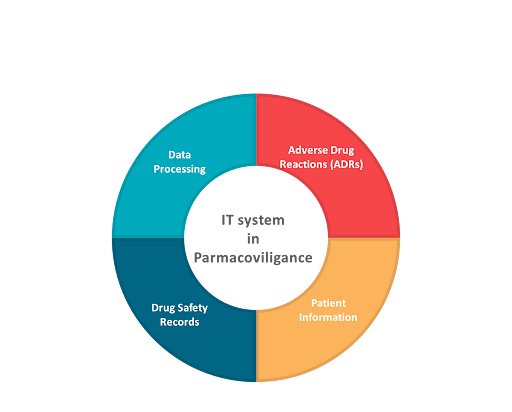
Additionally, resilient IT systems enhance data integration and sharing among stakeholders, including regulatory authorities, pharmaceutical companies, and healthcare professionals. This interconnectedness improves the overall effectiveness of pharmacovigilance activities by promoting transparency and collaboration. Robust IT systems are essential for maintaining regulatory compliance, as they ensure that all safety data is accurately reported and accessible for audits and inspections.
Widely used IT programmes
Softwares and programmes are commonly used in pharmacovigilance in order to ensure all data are safely and orderly recorded and analysed, some of them are:
- Oracle Argus Safety
- ArisG
- Oracle Adverse Event Reporting System (AERS)
- ClinTrace
- PvNET
- repClinical
- Vigilanz Dynamic Monitoring System
- WebVDME Pharmacovigilance Signal detection and Signal management software
- PV works
| Platforms | Key features |
Oracle Argus Safety  | * Ensure global regulatory compliance * Make faster, science-based safety decisions * Integrate safety and risk management * Lower the cost of Pharmacovigilance |
ArisG | * Maintain critical drug safety data in ARISg * Provides all the functionality required to manage adverse event reporting and adverse reaction requirements of different regulatory authorities |
Oracle AERS | * Supports the capture, management, reporting and analysis of serious adverse event and product compliance cases for all medical products including drugs, medical devices, vaccines, biologics and gene therapies from all clinical and spontaneous sources. * Provides and interactive presentation of key case information |
PvNET | * Extensive data validation and cross-field validation checks – validate case files for E2B compliance. * Global Dictionary support and Dictionary management (MedDRA version management) * Audit records for safety data management activities * Serious Adverse event automated narrative writing & multilingual text support |
Table 1. Detailed explanation of the uses and the key features of some pharmacovigilance systems
Impact of IT Outage on Pharmacovigilance
In Malta, as in other EU countries, an IT outage can lead to non-compliance with these regulations if ADR reports are delayed or if data cannot be accessed for audits and inspections. Such breaches can result in legal penalties and undermine public trust in the safety monitoring system. The recent MHRA GPvP symposium highlighted the critical nature of maintaining robust IT systems to ensure continuous pharmacovigilance operations across Europe.
Conclusion
Reliable IT systems are vital for effective pharmacovigilance. IT outages pose significant risks, including delays in reporting and potential data loss, which can impact patient safety. Stakeholders should invest in resilient IT systems to maintain continuous monitoring and ensure the safety of pharmacovigilance processes. As a leading consultancy in pharmaceutical regulatory affairs, Pharmaconsulta is dedicated to advocating for and implementing robust IT systems that support uninterrupted pharmacovigilance operations, ensuring the highest standards of drug safety and public health.
Pharmaconsulta is dedicated to advocating for and implementing robust IT systems that support uninterrupted pharmacovigilance operations, ensuring the highest standards of drug safety and public health.
Yun Shen