Falsified Medicines: A Growing Threat to Public Health
May 22, 2023The issue of falsified medicines has become a growing concern for public health authorities worldwide. Falsified medicines are those that are deliberately mislabelled, contain fake or low-quality ingredients, or are otherwise misrepresented as authentic medicines. They are a major threat to public health, as they can pose serious risks to patients’ health and well-being.The European Commission for Public Health has defined falsified medicines as those that are often disguised as authentic medicines but may contain ingredients of bad or toxic quality or may be in the wrong dosage. They have not been properly checked for quality, safety, and efficacy, as required by strict EU authorization, and can pose a real risk to patients’ health. As falsified medicines become more sophisticated, the risk of them reaching patients in the EU increases, representing a serious threat to global health.
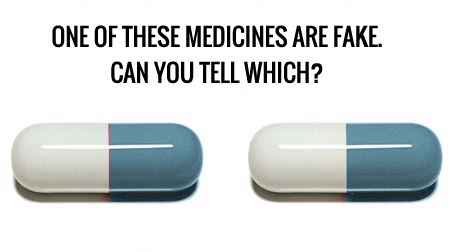
Meditpharma, as a pharmaceutical wholesaler, recognizes the seriousness of the issue and has taken steps to identify, avoid, and report falsified medicines. They have implemented strict procedures to combat the ever-evolving nature of the threat of falsified medicines. These procedures are constantly updated to reflect the latest developments in the field.Some markers of falsified medicine are obvious, such as an unusually low price, discolored or miscolored packages, offset or inconsistent text and labelling, and a general lack of quality control compared to legitimate, official products. Any products that exit the legal supply chain, be they stolen,expired, tampered with, or marked for export, are also considered falsified medicines. However, not all falsified medicines are so easily identifiable. Meditpharma takes its commitment to quality and safety seriously, and as part of that commitment, the company has established an extensive and meticulous due diligence process for all potential new suppliers or customers. This process begins with a structured onboarding procedure that is carefully designed to ensure that every potential client is thoroughly vetted before being approved. The experienced due diligence team reviews and confirms through the international and local databases the validity of the client’s licence, verification questionnaire, physical address, and legitimacy, leaving no stone unturned in their quest to ensure the safety and authenticity of the products being offered. Furthermore, Meditpharma requires all suppliers to submit six-sided high-resolution package photos of the products on offer. This step is essential to verifying that the product is genuine and that the batch numbers match the physical stock, thereby ensuring that every product sold by Meditpharma is of the highest quality and safety standards. By following these stringent procedures, Meditpharma is able to maintain its reputation for excellence and reliability in the pharmaceutical industry.
In order to ensure that all medicines sold by Meditpharma are authentic and safe, the company relies on the European Medicines Verification System (EMVS). This system, which is in place throughout the European Union, provides end-to-end verification of medicine authenticity and is available to all pharmacies and wholesalers. With the local branch of the EMVS, known as the Malta Medicines Verification Organisation (MAMVO), Meditpharma can remain vigilant against the serious threat of falsified medicines. MAMVO plays a crucial role in helping Meditpharma to maintain its commitment to quality and safety. Collaboration with MAMVO, Meditpharma is able to identify and remove any falsified medicines that may enter the supply chain.
In conclusion, falsified medicines are a growing threat to public health. They can pose serious risks to patients’ health and well-being and represent a significant challenge to the pharmaceutical industry. It is essential that pharmaceutical wholesalers and distributors take measures to identify, avoid, and report falsified medicines to combat this threat.

Meditpharma Ltd, 71, Triq Karmenu Camilleri, Qormi, QRM 4631, Malta.
Company Reg. C73939. VAT No. MT23064329. +356-79-990-020. Email: andrew@meditpharma.co